
A world where genes and data are used in preventing & treating diseases
Designing New Humans Through Gene Therapy
Gene therapy is a form of medical treatment that cures genetic diseases at their source by editing the patient’s genes to reinforce gene expression in cells or provide cells with new functionalities. Thanks to a recent drop in the cost of gene analysis, light has been shed on the mechanisms behind genetic diseases and led to a host of new treatments. In the face of drastic changes to healthcare, there have been calls for the need to discuss ethical issues resulting from progress in gene therapy technology. To provide some perspective on the situation, the Mitsubishi Research Institute has investigated the latest trends in research & development for rare diseases, cancer, and dementia. The findings will be shared over the course of this three-part series.
Present Situation and Issues of Gene Therapy
The year 2019 marked the beginning of a new era in gene therapy for Japan, with its pharmaceutical regulatory authority, the Pharmaceuticals and Medical Devices Agency (PMDA), approving two gene therapy drugs: Kymriah and Collategene. Kymriah is used to treat specific cases of relapsed or intractable leukemia, while Collategene is intended to treat critical lower-limb ischemia caused by peripheral artery disease. The two drugs will be covered by the national health insurance for patients in need of treatment, thus providing hope to patients with intractable disease. Although much welcomed, do these gene therapies present any concerns?
Let’s take a look at the current situation surrounding gene therapy.
Currently approved forms of gene therapy, including the use of Kymriah and Collategene, are designed for somatic cells. Cells are roughly divided into somatic and germ cells. Somatic cells form the body and only live for one generation whereas roughly germ cells produce a new individual from an embryo, basically a fertilized egg, and are passed on from generation to generation.
Presently, germ cell gene therapy has yet to be approved. What awaits if gene therapy can be provided for germ cells or on embryos? First, we will be able to treat hereditary genetic disorders. If a parent’s genetic test reveals any mutations, the application of gene therapy to eggs fertilized in-vitro will enable the mother to have a child free of the same mutation.
The enhancement of an individual’s abilities may also be possible with the application of gene modification beyond therapeutic purposes. In this case, “enhancement” refers to the medical intervention of the human mind and body to improve an individual’s abilities and disposition beyond the purpose of restoring and maintaining health. For example, increasing the expression of the erythropoietin (EPO) gene related to oxygen transport may help enhance muscle endurance. In another example, increasing the expression of the cAMP response element-binding protein (CREB) gene related to long-term memory formation in neurons may help enhance memory.
Current law prohibits the use of gene therapy on embryos for the purpose of giving birth. Although technological progress has been made, new technologies have yet to be proven safe and effective. Also, sufficient discussion has yet to be made regarding the surrounding ethical issues. Such ethical concerns include questions regarding the appropriate response when abnormalities are found during the growth of a child born from an embryo subject to gene therapy, and the possibility of discrimination and eugenics due to the spread of gene therapy for embryos in the future. Of particular importance is the need to consider the impact on future generations. Once gene editing is performed on an embryo, the modified genes are passed on semi-permanently to subsequent generations. Research must be preceded by careful discussion.
Inheritance of DNA Sequence Subject to Genome Editing
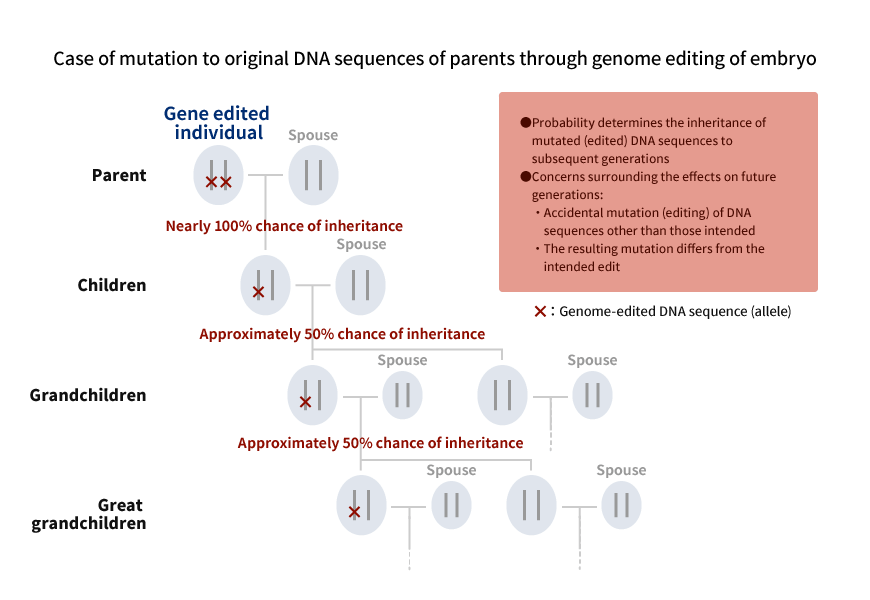
Source: Mitsubishi Research Institute, Inc.
Research for the Sake of Technological Advancement and Lagging Ethical Discussion
Discussions are currently underway among experts in the field on whether to allow the application of gene therapy to embryos solely for the purpose of research. The precision of genome editing is improving day by day. In December 2019, a team led by Tomoji Mashimo, Professor at the University of Tokyo, announced their successful development of a CRISPR/Cas3 genome-editing technology. They proved that the probability of CRISPR/Cas3 editing a location other than the target is equal to or lower than that of the representative genome-editing tool (CRISPR/Cas9). The CRISPR/Cas3 technology has high potential for future application.
The genome editing process itself does not require particularly difficult operations or large-scale equipment. For example, the process can be done in a university laboratory or infertility treatment clinic. However, genome editing does require a vigorous debate and a robust framework. In a situation where technological advancement was not accompanied by discussion and ethical regulation, He Jiankui, Associate Professor at the Southern University of Science and Technology in China, shocked the world by announcing in November 2018 that he had conducted gene therapy on embryos in vitro, resulting in the birth of twins. The university was not aware of the situation because Jiankui had made false statements in documents he submitted to the university’s ethics committee. Given that sufficient examination had not been performed before the embryo’s implantation to the mother, there was a lack of certainty as to whether the gene had been properly edited, and there was a possibility that unintended mutations could have occurred. Since gene therapy does not involve particularly difficult operations as mentioned above, cases similar to the one in China may have taken place elsewhere.
Issues Raised by Birth of Twins in China Through Genome Editing
Following the aforementioned case in China, we are certain that there are at least two individuals in the world who have received gene therapy during the germinal stage. We can only hope that the twin girls born in China will grow up healthy, without any problems in their follow-up studies. However, the field of gene therapy could come to a screeching halt if any physical problems suspected to have resulted from gene therapy are discovered in the twins.
In response to the incident in China, developers of genome editing technologies have “call[ed] for a global moratorium on all clinical uses of human germline editing…” (Lander et al.). The scientific community is concerned that the haphazard editing of the genes in an embryo may cause unexpected harm to patients, resulting in loss of public confidence in scientists.
Some countries enforce laws and regulations whereas others restrain research by discontinuing research funds. Japan is currently lagging in its own regulations, possibly due to concerns that the establishment of laws or penalties could hinder the research and application of gene therapy. Unless the Japanese government assumes a stance, however, the international community may inevitably view Japan as approving gene therapy for embryos. In light of the case of the twins in China and relatively low technological barriers to genome editing, Japan must hasten in developing its own regulations.
Gene therapy has raised new issues that had not existed in conventional pharmaceutical products. With the development of safe forms of genome editing technologies imminent, discussion must be carried out among all members of society on the pros and cons all forms of its use including medical treatment, wider-health purposes, and human enhancement. The Mitsubishi Research Institute, a company committed to co-creating the future, aims to continue analyzing trends in the field of gene therapy, researching the accompanying technological and societal issues, and sharing our findings with society.
[Reference]
1. Eric Lander et al. (2019) “Adopt a moratorium on heritable genome editing” nature
https://www.nature.com/articles/d41586-019-00726-5 (Accessed: February 25, 2020)
