- TOP
- Columns
- Can Genomic Medicine Be a Beacon of Hope in Tests and Treatments for Alzheimer’s Disease?

A world where genes and data are used in preventing & treating diseases
Can Genomic Medicine Be a Beacon of Hope in Tests and Treatments for Alzheimer’s Disease?
Medical Therapy for Alzheimer’s Disease: Hope and Reality
In late October 2019, Biogen and Eisai Co., Ltd announced their intention to submit an application for the approval of aducanumab, a candidate drug for the treatment of Alzheimer’s disease, to the United States Food and Drug Administration (FDA). While up to now giants in the pharmaceutical industry have undertaken research and development of medications for treating Alzheimer’s disease, clinical trials have been successively terminated. Given the general view to date that dementia is an untreatable disease, the announcement by Biogen and Eisai Co., Ltd. was undoubtedly received with great excitement by many.
The deposition of a peptide known as Amyloid-β in areas surrounding neurons in the brain and mutations of tau proteins, which likewise exist within neurons, and their accumulation in the neuronal cells of the central nervous system were cited as the likely causes of Alzheimer’s disease. Until now, many medications containing aducanumab were developed with the aim of eliminating Amyloid-β or suppressing its synthesis. However, drug development stalled due to the fact that Amyloid-β deposition begins 10 to 15 years prior to the onset of Alzheimer’s disease*1 and because the mechanism of action of the medications differed in humans and mice, making it difficult to replicate the effects of clinical trials using mice in humans.
Given the current state of affairs, research and development of medications aimed at protecting neurons or suppressing neuronal cell death or inflammation have quickly gathered momentum. In 2019, about 60% of phase III clinical trials (large-scale clinical trials aimed at verifying the efficacy or safety of drugs) featured drugs with “other targets” besides Amyloid-β or tau proteins.*2 The drug-treatment strategy for Alzheimer’s disease is now moving away from eliminating the causes of Alzheimer’s towards improving and protecting brain functions.
Drug-treatment strategies for Alzheimer’s disease
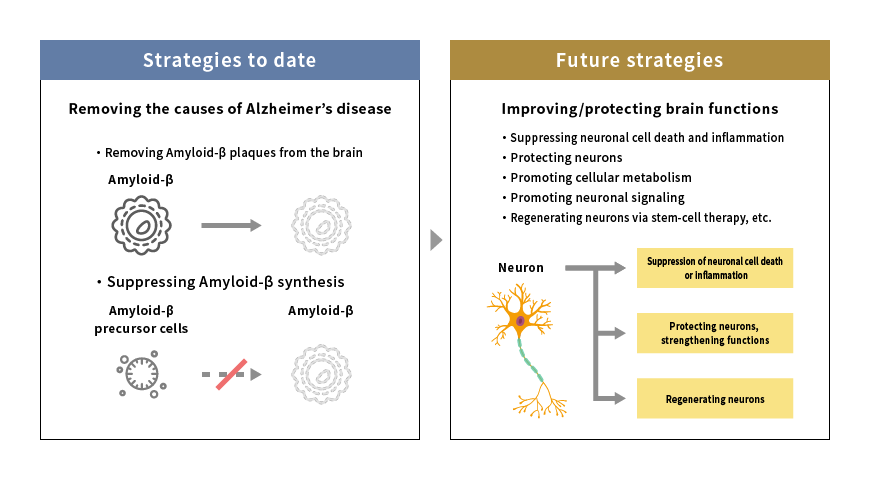
Source: Mitsubishi Research Institute, Inc.
Screening for Alzheimer’s Disease Using Genetic Data
How can we expect genomic medicine to contribute to tests and treatments for Alzheimer’s disease?
The previous column described the increasing use of genetic analysis in screening tests and gene therapy for rare diseases and oncology areas against the background of improvements in gene-editing technology and the decreasing cost of genetic analysis.
The same trends are occurring in the field of Alzheimer’s disease research, with particularly strong hopes pinned on screening methods that employ genetic data.
Current screening methods for dementia involve using cognitive function tests through questionnaires, CT or MRI imaging studies or blood and cerebrospinal fluid analysis.*3 However, these screening methods are not ideal, since they become useful only after symptoms of cognitive impairment have already appeared. Because Amyloid-β begins accumulating in the brain 10 to 15 years prior to the onset of Alzheimer’s disease, screening and treatment should ideally begin before symptom onset in order to maximize the efficacy of treatment. Moreover, imaging studies are costly to perform, and cerebrospinal fluid analysis is a highly invasive procedure with the potential for significant stress and adverse bodily effects. In view of the ongoing rise in the number of patients, an inexpensive and minimally invasive screening procedure is highly desirable.
Given these circumstances, attention has increasingly focused on research on the relationship between Alzheimer’s disease and genetic data, especially blood microRNA (miRNA). If the biomarkers in blood miRNA are identified, a simple and inexpensive blood screening test deployable on a large scale will become possible, enabling early detection even in the absence of signs of cognitive impairment. Presently, factors related to oxidative stress in the brain that are contained within miRNA and factors modulating the cell cycle have been cited as candidate biomarkers of Alzheimer’s disease,*4 raising hopes for the development of new testing methods. (Figure 2)
Changes in Alzheimer’s disease testing methods
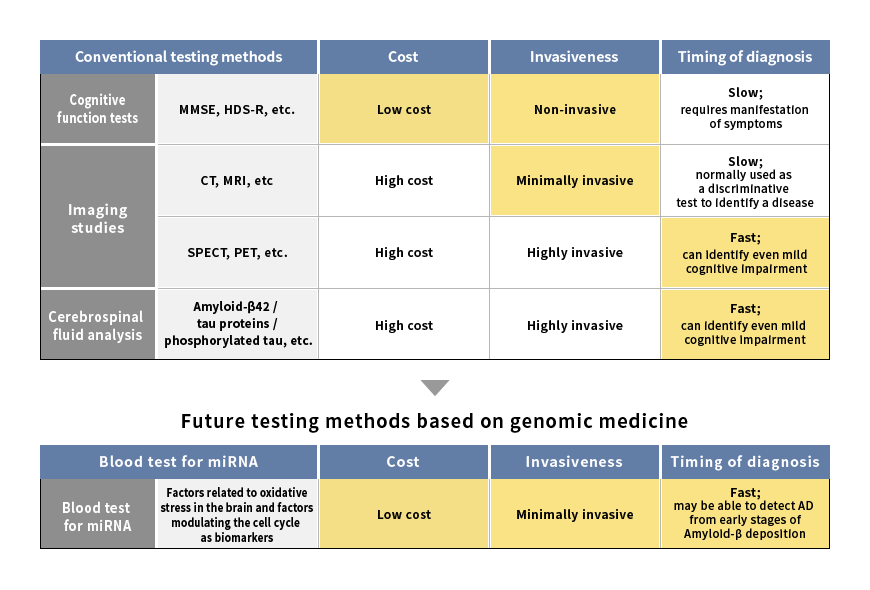
Source: Mitsubishi Research Institute, Inc.
Further, the National Center for Geriatrics and Gerontology has created a model to predict the risk of developing dementia based on blood miRNA,*5 which has brought to light the possibility of developing technologies not only for new screening methods, but also for risk prediction.
Progress in biomarker technologies, such as those using blood miRNA, is also likely to greatly influence the course of drug research and development. Current clinical drug trials for dementia treatment target improvements in cognitive functions and lack the necessary criteria to assess the disease state, such as the biomarkers or tumor excisions used in assessing cancer. Advances in biomarker technologies will enable neuropathies to be assessed based on fluctuations in biomarker levels and open the way to newer technologies and research. Furthermore, establishing biomarkers will broaden our ability to identify persons with dementia risk before symptom onset and assess treatment efficacy more quickly while also providing impetus to the development of treatment methods.
The Potential for Gene Therapy for Alzheimer’s Disease
Progress in genomic medicine is demonstrating the potential for novel forms of genetic therapy, such as aducanumab, that differ in their approach from conventional medical treatments of Alzheimer’s disease.
The Institute of Physical and Chemical Research (RIKEN) demonstrated that the accumulation of Amyloid-β can be suppressed by deleting a specific gene region in mice using genome editing technology.*6 Further, a phase I clinical trial of a gene therapy using APOE2, which is known to reduce the risk of developing Alzheimer’s disease, is currently underway.*7 Biogen and Sangamo Therapeutics announced that they have joined forces to develop gene therapy for various diseases, including Alzheimer’s,*8 raising hopes for advances in gene therapy for Alzheimer’s.
There are concerns and challenges as well. The discovery of neurodegenerative changes in wide areas of brains with Alzheimer’s disease, including the hippocampus and cerebral cortex, and the involvement of factors related to Amyloid-β in the mediation of brain functions besides Amyloid-β synthesis suggest that treatments targeting specific genes may not be effective. Furthermore, in gene therapy a virus vector needs to be developed to deliver genetic materials to cells while the cost of developing and establishing the safety of such a delivery system poses a distinct challenge. Additionally, gene therapy is generally very expensive; therefore, given the large number of Alzheimer’s disease patients worldwide, the impact of this treatment on medical costs and the insurance system needs to be weighed simultaneously.
Genomic Medicine Paves the Way for Alzheimer’s Disease Treatment
As seen thus far, progress in genomic medicine has significantly influenced screening for Alzheimer’s disease, leading to predictions that a cheap, simple, and convenient test will become available in the near future. Further, the identification of biomarkers contains the potential for a paradigm shift in the development of therapeutic agents, and gene therapy for Alzheimer’s is likely to receive increasing research attention.
However, it appears that more time will be needed before everyone is able to enjoy the fruits the screening and treatment methods afforded by genome medicine. What is more, even if progress in screening technology enables early diagnosis, the absence of a radical treatment could cause distress in patients with dementia. Thus, support for such individuals is also needed.
For these reasons, in tandem with these scientific developments, it is necessary to create a society where persons with dementia can lead their lives with peace of mind. For example, through the coordinated efforts of stakeholders, such as medical institutions and government, systems capable of caring for persons with dementia can be created along with assistance to such individuals in finding employment or expressing their opinions. An excellent example has been given by the townships of Machida in Tokyo and Omuta in Fukuoka Prefecture, where the local government has collaborated with persons with dementia to create a community that is friendly to those afflicted with this disease.
The number of patients with dementia is predicted to rise to 74,700,000 by 2030.*9 Let us continue to hope that not only will dementia be able to be diagnosed and treated before the onset of symptoms through new technologies, such as genomic medicine, but also that we will be able to realize a society where persons with dementia have the assurance of living with peace of mind.
- *1: Clifford R Jack Jr et at al., Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers, The Lancet Neurology 12(2) 207-216, 2013
- *2: Jeffrey Cummings et al., Alzheimer‘s disease drug development pipeline: 2019, Alzheimer’s & Dementia, 272-293, 2019
- *3: [2017 Treatment Guidelines for Dementia], Igakusho-in, 2017
- *4: Swarbrick, S., Wragg, N., Ghosh, S. et al. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol Neurobiol 56, 6156–6167, 2019
-
*5: [Construction of a risk prediction model for dementia symptom onset using miRNA] (press release of the Japan Agency for Medical Research and Development), February 25, 2019
https://www.amed.go.jp/news/release_20190225-03.html (Accessed: April 21, 2020) -
*6: [Preventing Alzheimer’s disease using gene editing: paving the way for the application of nucleic medicine] (press release of RIKEN) (May 4, 2018)
https://www.riken.jp/press/2018/20180504_2/index.html (Accessed: April 21, 2020) -
*7: ClinicalTrials.gov "Gene Therapy for APOE4 Homozygote of Alzheimer's Disease"
https://clinicaltrials.gov/ct2/show/NCT03634007 (Accessed: April 21, 2020) -
*8: Biogen News Releases "BIOGEN AND SANGAMO ANNOUNCE GLOBAL COLLABORATION TO DEVELOP GENE REGULATION THERAPIES FOR ALZHEIMER’S, PARKINSON’S, NEUROMUSCULAR, AND OTHER NEUROLOGICAL DISEASES", February 27, 2020
http://investors.biogen.com/news-releases/news-release-details/biogen-and-sangamo-announce-global-collaboration-develop-gene (Accessed: April 21, 2020) -
*9: Alzheimer’s Disease International "World Alzheimer Report 2015"
https://www.alz.co.uk/research/world-report-2015 (Accessed: April 21, 2020)
